You have no items in your shopping cart.
Biosimilars
Background
A biosimilar is defined as a biopharmaceutical product (also known as a biologic), which is highly similar to a pre-approved reference biological medicine. Biosimilars are approved according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological medicines set by drug regulatory authorities, such as EMA (EU) and the FDA (US). Unlike generic medicines manufactured via chemical synthesis, biologic medicines contain one or more active components derived from a biologic source, such as recombinant proteins. In contrast to the more common small molecule-based drugs, biologics usually exhibit high molecular complexity and can be sensitive to changes during the manufacturing processes.
- The EU was the first region in the world to establish a framework for the approval of biosimilar medicines.
- The EMA has granted authorization for over 50 biosimilars since 2006.
- The EU approved the first biosimilar of a monoclonal antibody based on infliximab.
- In 2015 the FDA approved the USA’s first biosimilar product, filgrastim-sndz.
Biologics versus Traditional Drugs
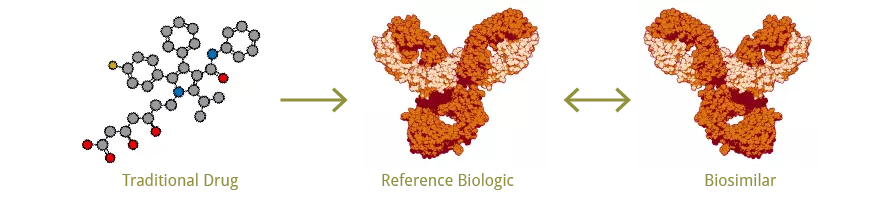
Fig. 1. Biologics versus Traditional Drugs
|
|
Manufacturing Biologics and Biosimilars
Biologics have been manufactured for decades since the development of human growth hormone, insulin, and red-blood cell stimulating agents. The library of potential targets has increased rapidly through the exponential growth in genomics research coupled with a greater understanding of subcellular cascades and disease pathology. Scientific fields used in developing biologics include genomics and proteomics, as well as microarray, cell culture, and monoclonal antibody technologies. The therapeutic target of a biologic is always a gene or a protein. Recombinant DNA, an important component in the production of biologics, requires the isolation of DNA from cells and potentially modifying that DNA segment, inserting it into a bacterial or a mammalian cell host, and inducing expression.

Fig. 2. Steps in developing Biosimilars
Several steps are required in the development process:
All newly identified proteins of interest undergo a series of cell-based assays providing further information on their involvement in various biological processes. Bioassays are then performed to determine potency, utilizing common biological indicators in living organisms or tissues. These can include cell-based tissue cultures, microarray expression technology, knockout / transgenic animal models and anti-sense or antibody technology, such as diagnostic antibody characterization.
Biosimilars in Research
The future of biologics and biosimilars is clear; they are here to stay and their use in both clinical and research settings is rising. Due to the reduced approval process required of the FDA and EMA for biosimilars, these drugs are typically less expensive, by approximately one-half in some cases. From a research perspective, biosimilars offer a near identical alternative for the fraction of the cost of biologics. Using a biosimilar will reduce reagent costs without impacting the validity of your results. However, producing antibodies that reliably target biosimilars requires a wealth of experience and time that many researchers do not have. Biorbyt are proud to be able to offer a wide range of antibodies targeting the most commonly used biosimilars.
Sources
- European Medicines Agency (EMA)
- Food and Drug Administration (FDA)
- Advances in Drug Development- Current Developments in Oncology Drug Research: The Benefits and Drawbacks of Biosimilars- Bruce N. Cronstein, MD
- Morrow T, Felcone LH. Defining the difference: What Makes Biologics Unique. Biotechnol Healthc. 2004;1(4):24-29.
- Biosimilars: An overview (March 2011)
- DOI: 10.2147/BS.S16120